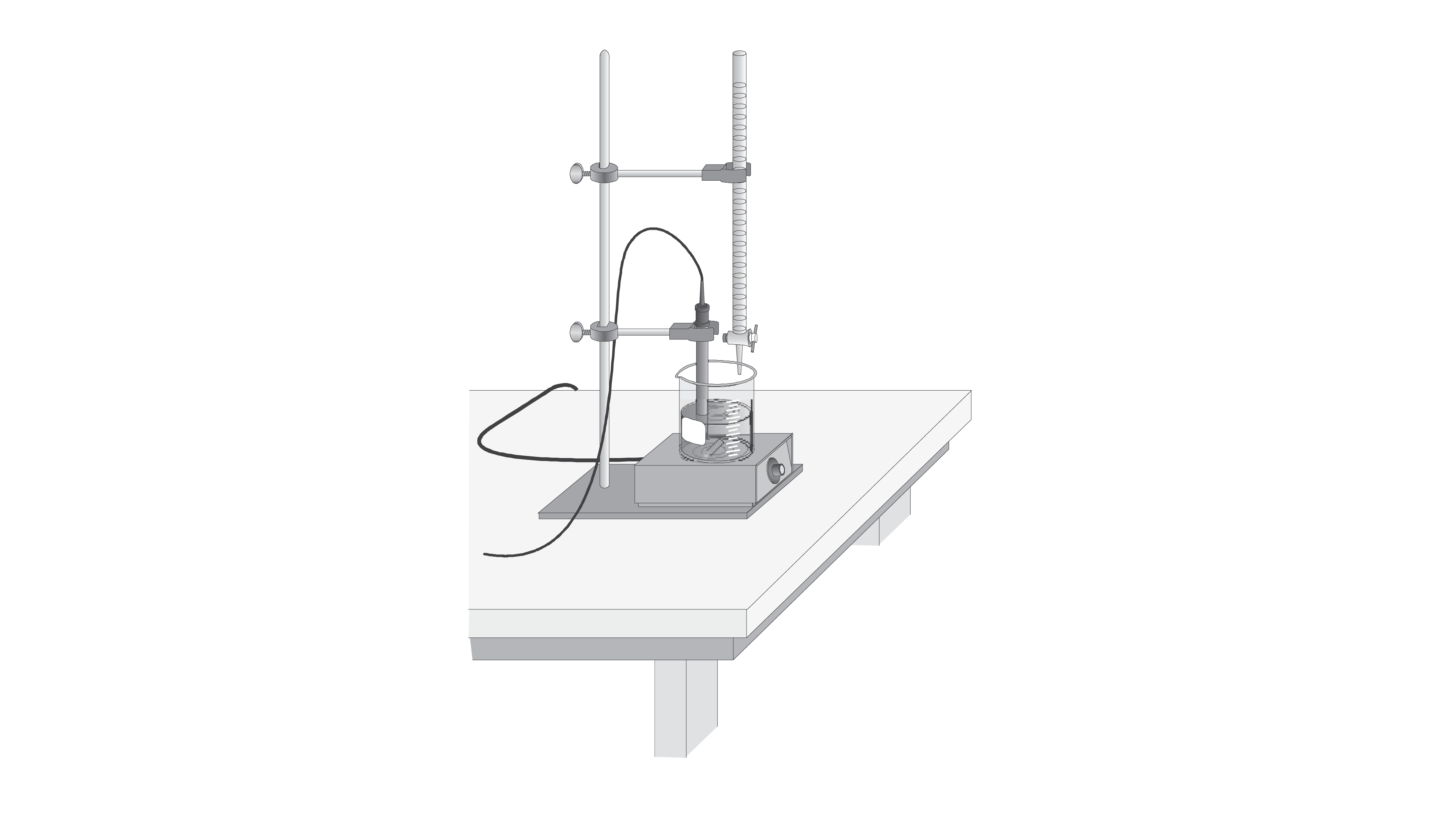
Acid-Base Titration Lab Advanced Chemistry With Vernier Answers . advanced chemistry with vernier. A titration is a process used to determine the volume of a. in this experiment, your goal is to determine the molar concentration of two acid solutions by conducting titrations with a base of known concentration. a titration is a process used to determine the volume of a solution needed to react with a given amount of another substance. A titration is a process used to determine the volume of a solution that is needed to react with a given. In this experiment, you will. calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m. A titration is a laboratory process used to determine the volume of a solution needed to react with a given.
from www.vernier.com
A titration is a process used to determine the volume of a. advanced chemistry with vernier. calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m. in this experiment, your goal is to determine the molar concentration of two acid solutions by conducting titrations with a base of known concentration. a titration is a process used to determine the volume of a solution needed to react with a given amount of another substance. In this experiment, you will. A titration is a process used to determine the volume of a solution that is needed to react with a given. A titration is a laboratory process used to determine the volume of a solution needed to react with a given.
AcidBase Titration > Experiment 7 from Advanced Chemistry with Vernier
Acid-Base Titration Lab Advanced Chemistry With Vernier Answers in this experiment, your goal is to determine the molar concentration of two acid solutions by conducting titrations with a base of known concentration. A titration is a process used to determine the volume of a solution that is needed to react with a given. A titration is a process used to determine the volume of a. A titration is a laboratory process used to determine the volume of a solution needed to react with a given. a titration is a process used to determine the volume of a solution needed to react with a given amount of another substance. advanced chemistry with vernier. in this experiment, your goal is to determine the molar concentration of two acid solutions by conducting titrations with a base of known concentration. calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m. In this experiment, you will.
From www.chegg.com
Solved AcidBase Titration Virtual Lab Introduction In Acid-Base Titration Lab Advanced Chemistry With Vernier Answers A titration is a process used to determine the volume of a. calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m. A titration is a laboratory process used to determine the volume of a solution needed to react with a given. in this experiment, your goal. Acid-Base Titration Lab Advanced Chemistry With Vernier Answers.
From studylib.net
acid base titration worksheet answer key Acid-Base Titration Lab Advanced Chemistry With Vernier Answers A titration is a process used to determine the volume of a. in this experiment, your goal is to determine the molar concentration of two acid solutions by conducting titrations with a base of known concentration. A titration is a laboratory process used to determine the volume of a solution needed to react with a given. A titration is. Acid-Base Titration Lab Advanced Chemistry With Vernier Answers.
From yazminewadiaz.blogspot.com
Acid Base Titration Lab Report Answers YazminewaDiaz Acid-Base Titration Lab Advanced Chemistry With Vernier Answers A titration is a process used to determine the volume of a. in this experiment, your goal is to determine the molar concentration of two acid solutions by conducting titrations with a base of known concentration. A titration is a process used to determine the volume of a solution that is needed to react with a given. calculate. Acid-Base Titration Lab Advanced Chemistry With Vernier Answers.
From www.vernier.com
AcidBase Titration > Experiment 24 from Chemistry with Vernier Acid-Base Titration Lab Advanced Chemistry With Vernier Answers in this experiment, your goal is to determine the molar concentration of two acid solutions by conducting titrations with a base of known concentration. In this experiment, you will. a titration is a process used to determine the volume of a solution needed to react with a given amount of another substance. A titration is a process used. Acid-Base Titration Lab Advanced Chemistry With Vernier Answers.
From www.chegg.com
Solved REPORT SHEET LAB AcidBase Titration 20 A. Acid-Base Titration Lab Advanced Chemistry With Vernier Answers A titration is a laboratory process used to determine the volume of a solution needed to react with a given. A titration is a process used to determine the volume of a solution that is needed to react with a given. calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid). Acid-Base Titration Lab Advanced Chemistry With Vernier Answers.
From www.vernier.com
AcidBase Titration > Experiment 7 from Advanced Chemistry with Vernier Acid-Base Titration Lab Advanced Chemistry With Vernier Answers advanced chemistry with vernier. in this experiment, your goal is to determine the molar concentration of two acid solutions by conducting titrations with a base of known concentration. a titration is a process used to determine the volume of a solution needed to react with a given amount of another substance. A titration is a process used. Acid-Base Titration Lab Advanced Chemistry With Vernier Answers.
From soetrust.org
2022 UPDATED!!! Acid base titration lab answers Soetrust Acid-Base Titration Lab Advanced Chemistry With Vernier Answers A titration is a process used to determine the volume of a solution that is needed to react with a given. advanced chemistry with vernier. calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m. A titration is a process used to determine the volume of a.. Acid-Base Titration Lab Advanced Chemistry With Vernier Answers.
From mungfali.com
Acid Base Titration Experiment Acid-Base Titration Lab Advanced Chemistry With Vernier Answers advanced chemistry with vernier. calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m. A titration is a process used to determine the volume of a solution that is needed to react with a given. In this experiment, you will. a titration is a process used. Acid-Base Titration Lab Advanced Chemistry With Vernier Answers.
From 2021notubenju.blogspot.com
[11+] Acid Base Review Answers Important Questions Of Acid And Bases Acid-Base Titration Lab Advanced Chemistry With Vernier Answers A titration is a laboratory process used to determine the volume of a solution needed to react with a given. A titration is a process used to determine the volume of a. in this experiment, your goal is to determine the molar concentration of two acid solutions by conducting titrations with a base of known concentration. a titration. Acid-Base Titration Lab Advanced Chemistry With Vernier Answers.
From www.chegg.com
Solved LAB REPORT SHEET AcidBase Titration 10 A. Acid-Base Titration Lab Advanced Chemistry With Vernier Answers a titration is a process used to determine the volume of a solution needed to react with a given amount of another substance. in this experiment, your goal is to determine the molar concentration of two acid solutions by conducting titrations with a base of known concentration. advanced chemistry with vernier. A titration is a process used. Acid-Base Titration Lab Advanced Chemistry With Vernier Answers.
From chem.libretexts.org
AcidBase Titrations Chemistry LibreTexts Acid-Base Titration Lab Advanced Chemistry With Vernier Answers advanced chemistry with vernier. A titration is a process used to determine the volume of a. A titration is a laboratory process used to determine the volume of a solution needed to react with a given. calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m. . Acid-Base Titration Lab Advanced Chemistry With Vernier Answers.
From studylib.net
Acid/Base Titration Lab Acid-Base Titration Lab Advanced Chemistry With Vernier Answers A titration is a laboratory process used to determine the volume of a solution needed to react with a given. A titration is a process used to determine the volume of a solution that is needed to react with a given. a titration is a process used to determine the volume of a solution needed to react with a. Acid-Base Titration Lab Advanced Chemistry With Vernier Answers.
From www.chegg.com
Solved LAB REPORT SHEET AcidBase Titration 12 . Acid-Base Titration Lab Advanced Chemistry With Vernier Answers calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m. A titration is a laboratory process used to determine the volume of a solution needed to react with a given. advanced chemistry with vernier. A titration is a process used to determine the volume of a. . Acid-Base Titration Lab Advanced Chemistry With Vernier Answers.
From www.vrogue.co
Acid Base Titration Lab Answers vrogue.co Acid-Base Titration Lab Advanced Chemistry With Vernier Answers A titration is a laboratory process used to determine the volume of a solution needed to react with a given. A titration is a process used to determine the volume of a solution that is needed to react with a given. A titration is a process used to determine the volume of a. advanced chemistry with vernier. a. Acid-Base Titration Lab Advanced Chemistry With Vernier Answers.
From www.studeersnel.nl
Acid Base Lab lactic acid titration lab report Acid Base Titrations Acid-Base Titration Lab Advanced Chemistry With Vernier Answers A titration is a process used to determine the volume of a solution that is needed to react with a given. In this experiment, you will. calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m. A titration is a process used to determine the volume of a.. Acid-Base Titration Lab Advanced Chemistry With Vernier Answers.
From www.studypool.com
SOLUTION Acid Base Titration Datasheet Studypool Acid-Base Titration Lab Advanced Chemistry With Vernier Answers A titration is a laboratory process used to determine the volume of a solution needed to react with a given. In this experiment, you will. in this experiment, your goal is to determine the molar concentration of two acid solutions by conducting titrations with a base of known concentration. A titration is a process used to determine the volume. Acid-Base Titration Lab Advanced Chemistry With Vernier Answers.
From www.docsity.com
Acid base titration lab answers Docsity Acid-Base Titration Lab Advanced Chemistry With Vernier Answers In this experiment, you will. calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m. A titration is a laboratory process used to determine the volume of a solution needed to react with a given. in this experiment, your goal is to determine the molar concentration of. Acid-Base Titration Lab Advanced Chemistry With Vernier Answers.
From www.coursehero.com
[Solved] Lab report on Acid base titration Course Hero Acid-Base Titration Lab Advanced Chemistry With Vernier Answers calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m. In this experiment, you will. A titration is a laboratory process used to determine the volume of a solution needed to react with a given. A titration is a process used to determine the volume of a. . Acid-Base Titration Lab Advanced Chemistry With Vernier Answers.